Description
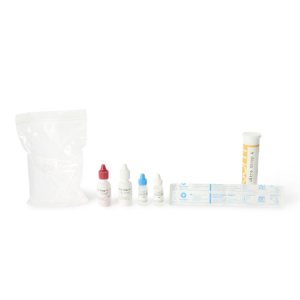
The Assure COVID-19 IgG/IgM Rapid Test Device is for use under FDA Emergency Use Authorization: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations. Use of this test with all authorized specimen types is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform moderate or high complexity tests; this test is also authorized for use with fingerstick whole blood specimens only at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. Assure COVID-19 IgG/IgM Control is intended to be used as external quality control material for the Assure COVID-19 IgG/IgM Rapid Test Device; the control can be used to verify proper performance of COVID-19 IgG/IgM Rapid Test kit. The Assure COVID-19 IgG/IgM Rapid Test Device is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection - at this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities. False positive results for Assure COVID-19 IgG/IgM Rapid Test Device may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using second, different IgG or IgM assay. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. Negative controls are lyophilized human serum samples and positive controls are lyophilized IgG and IgM against SARS-CoV-2. 2 Vials Neg / 1 Vial Pos allow for total 5 positive QC tests and 5 negative QC tests
Specification
- Application : Control Kit
- Container Type : Vial
- For Use With : For use with Assure COVID-19 IgG / IgM Assay
- Form : Powder
- Levels : Positive Level / Negative Level
- Product Dating : McKesson Acceptable Dating: we will ship >= 30 days
- Storage Requirements : USP Controlled Room Temperature
- Test Name : COVID-19 IgG / IgM
- Test Type : Antibody Test
- UNSPSC Code : 41116128
Attachment : Product Specification Sheet
| Vendor | McKesson |
| Price Range | $51 - $100 |
| Shipping Cost |
|
| Shop Location | Irving, Texas (TX), United States (US) |
No reviews found!
 English
English








































































































































No comments found for this product. Be the first to comment!