Description
BinaxNOW RSV Card is a rapid immunochromatographic assay for the qualitative detection of respiratory syncytial virus (RSV) fusion protein antigen in nasal wash and nasopharyngeal swab specimens from symptomatic patients. Shelf Life: 24 months from the date of manufacture. Retrospective nasal wash Sensitivity: 89% Specificity: 100%. Prospective NP swab Sensitivity: 93% Specificity 93%. This test is intended for in vitro diagnostic use to aid in the diagnosis of respiratory syncytial virus infections in neonatal and pediatric patients under the age of 5
Specification
- Application : Rapid Test Kit
- CLIA Classification : CLIA Waived
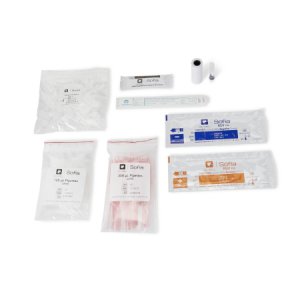
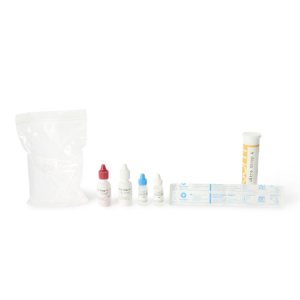
- Contents 1 : (10) Test Cards, Transfer Pipettes, Positive Control Swab (Inactivated RSV), Negative Control Swab, Elution Solution and NP Patient Swabs (with CLIA Waived Kits), Product Insert, Procedural Card
- Number of Tests : 10 Tests
- Product Dating : McKesson Acceptable Dating: we will ship >= 90 days
- Reading Type : Visual Read
- Sample Type : Nasopharyngeal Swab / Nasal Wash Sample
- Test Format : Test Card Format
- Test Method : Lateral Flow Method
- Test Name : Respiratory Syncytial Virus Test (RSV)
- Test Type : Infectious Disease Immunoassay
- Time to Results : 15 Minute Results
- UNSPSC Code : 41116144
Attachment : Product Specification Sheet
| Vendor | McKesson |
| Rango de precios | $101 - $200 |
| Costo de envío |
|
| Ubicación de la tienda | Irving, Texas (TX), United States (US) |
No se encontraron opiniones!
 Spanish
Spanish















































































































































No se han encontrado comentarios para este producto. ¡Sé el primero en comentar!